The Beer-lambert Law Is a Linear Relationship for
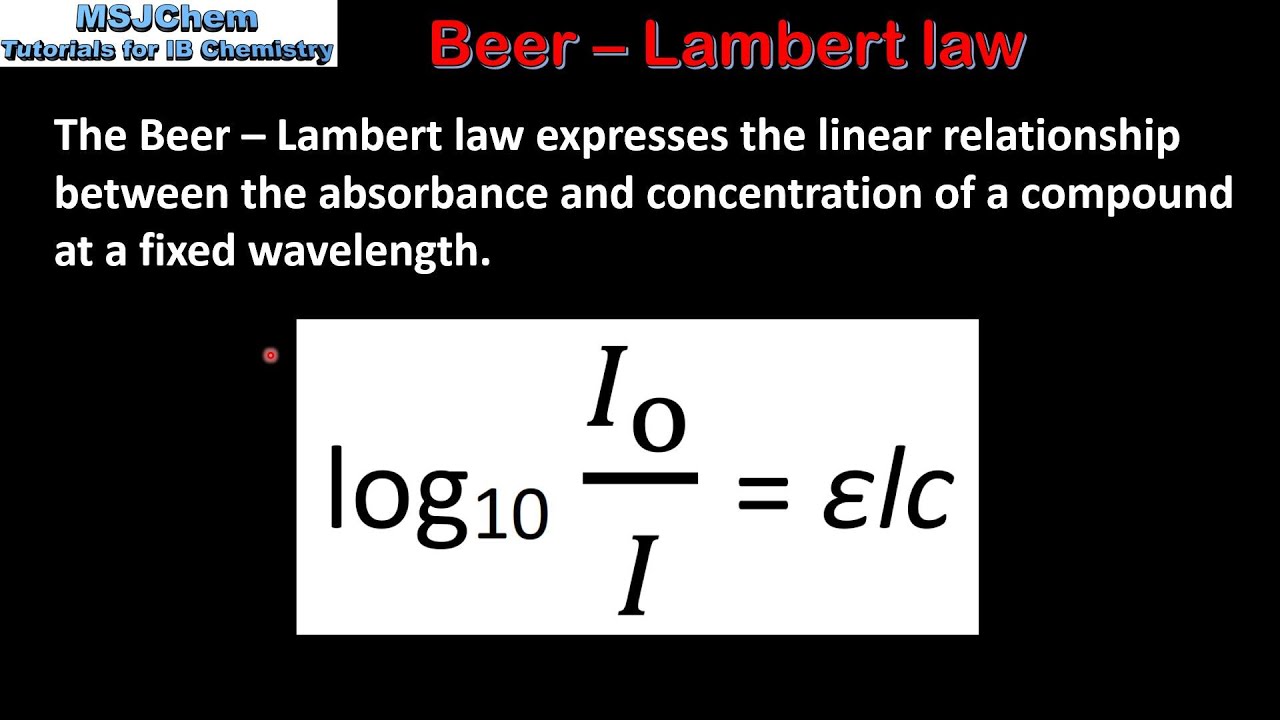
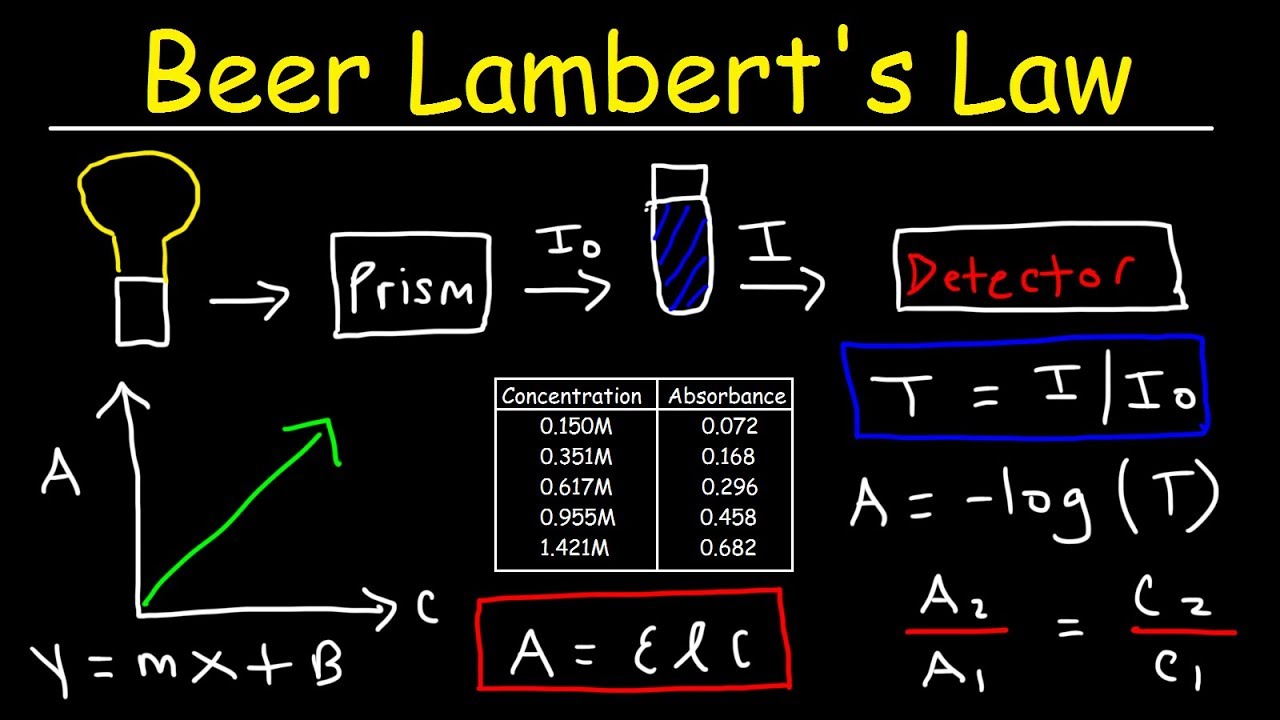
Beer Lambert Law explained there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its absorbance. The Beer-Lambert law is a linear relationship for Select one O a.
Why Is The Relation Between Concentration And Absorption Linear Based On Beer S Law Quora
OA is the absorbance of the solution Ab.

. The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its absorbance. Where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration. Beer-Lambert Law is the linear relationship between absorbance and concentration of an absorbing species.
A a b c. Impure compounds in inert solvents. Pure compounds in inert solvents.
In the early 1960s the connection between dispersion theory and Beers law was known7 However due to the use of the simplification introduced by Lorentz in 19068 known as the Lorentzprofile9 instead of a damped harmonic oscillator Lorentzoscillator a linear dependence of absorbance from concentration was found which. Pure compounds in reactive solvents. To determine if the Beer-Lambert Law is obeyed over a given concentration range by a given species measure absorbance as a function of concentration using the same test-tube for all of the measurements.
Common law is a system by which a court. The linear relationship between concentration and absorbance is both simple and straightforward which is why we prefer to express the Beer-Lambert law using absorbance as a measure of the absorption rather than T. The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its absorbance.
Pure compounds in inert solvents d. Pure compounds in inert solvent O b. The Beer-Lambert law is a linear relationship for Select one.
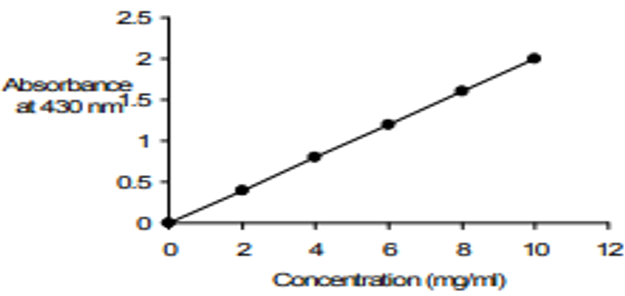
Check the linear nature of the curve. This is the reason that the attenuation coefficient also depends on concentration. What is the significance of the molar absorptivity e.
The Beer-Lambert law is a linear relationship for. The Beer Lambert law is a linear relationship between the concentration and absorbance optical coefficient and molar absorption coefficient of a solution. A a m x c x l Where.
Pure compounds in reactive solvents. The Beer-Lambert law or Beers law is the linear relationship between absorbance and concentration of an absorbing species. The absorption of light by a solution is described by the Beer-Lambert law as.
The general Beer-Lambert law is usually written as. O a m the molar extinctionabsorption coefficient. A E The View the full answer Transcribed image text.
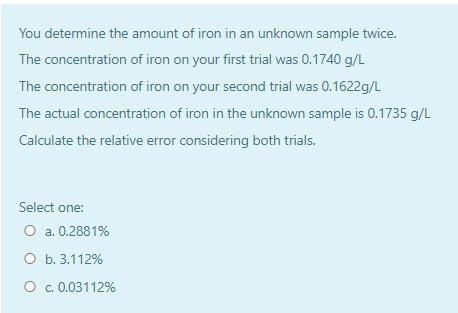
The concentration of iron on your first trial was 01740 gL The. The Beer-Lambert law is a convenient means to calculate the results of spectroscopic experiments eg the concentration of the absorbing species the extinction coefficient of the absorbing substance etc. The Beer-Lambert law is the linear relationship between absorbance and concentration of an absorbing species.
In accordance with the Beer-Lambert law absorbance is proportional to concentration and optical path length of the absorbers in the sample and in a linear relationship with total column concentration product of concentration and optical path length at a. From this it is clear that the concentrations of the light absorbers in tissue. Also Know what is beer Lambert law in chemistry.
Impure compounds in reactive solvents upport613-520-3700 or itsservicedeskcarletonca. A a b c. A a b c.
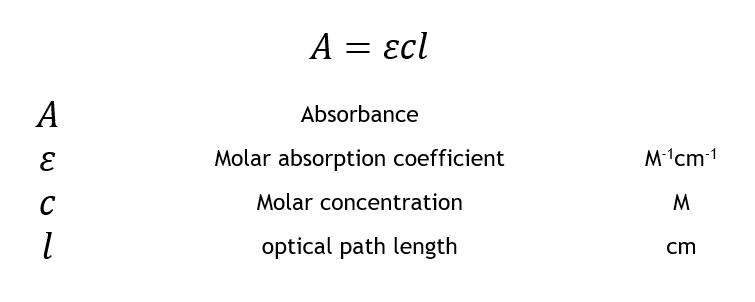
The Beer-Lambert law states that there is a linear relationship between the concentration and the absorbance of the solution which enables the concentration of a solution to be calculated by measuring its absorbance. The Beer-Lambert law or Beers law is the linear relationship between absorbance and concentration of an absorbing species. Where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration.
A a b c. Impure compounds in inert solvents O d. You might be interested.
Impure compounds in inert solvents c. The Beer-Lambert law or Beers law is the linear relationship between absorbance and concentration of an absorbing species. The general Beer-Lambert law is usually written as.
There is linear relationship between absorbance and concentration of an absorbing species. As mentioned previously the Beer-Lambert law defines that the light attenuation through a medium A λ is proportional to the concentration of the light absorbers present in the substance C K the optical properties of the light absorber ε Kλ and the optical pathlength traveled by the light beam L. To begin we will rearrange the equation A e bc.
The general Beer-Lambert law is usually written as. Presently the Beer lambert law is declared as a limiting law because the absorbance is only nearly linear depending on the concentration. Impure compounds in reactive solvents O c.
Where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration. Pure compounds in reactive solvents You determine the amount of iron in an unknown sample twice. Furthermore the Beer Lambert law states that a linear relationship exists between the absorbance and concentration of the solution.
The general Beer-Lambert law is usually written as. Moreover this relationship makes possible the calculation of the concentration of a solution by. Where A is the measured absorbance a is a wavelength-dependent absorptivity coefficient b is the path length and c is the analyte concentration.
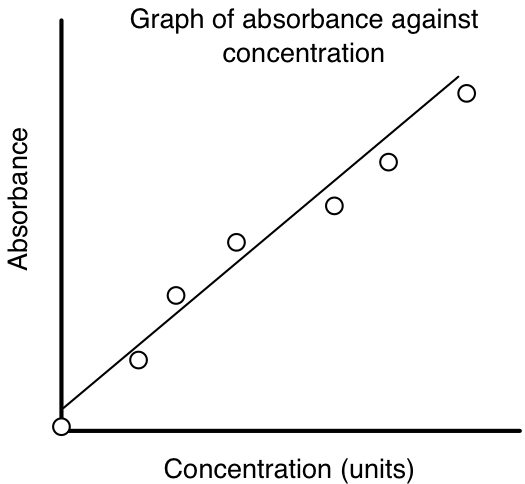
Beer Lambert Law The School Of Biomedical Sciences Wiki
Solved The Beer Lambert Law Is A Linear Relationship For Chegg Com

Solved The Beer Lambert Law Is A Linear Relationship For Chegg Com
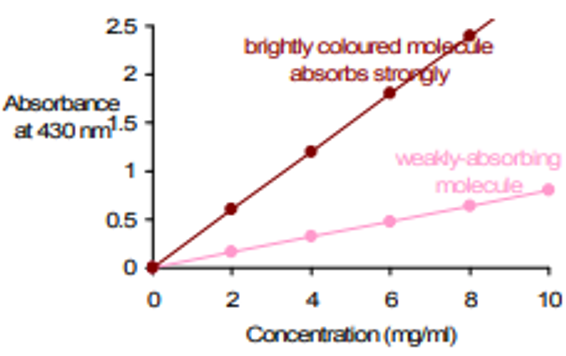
Beer Lambert Law History Definition Example Calculation
Beer S Law Theoretical Principles
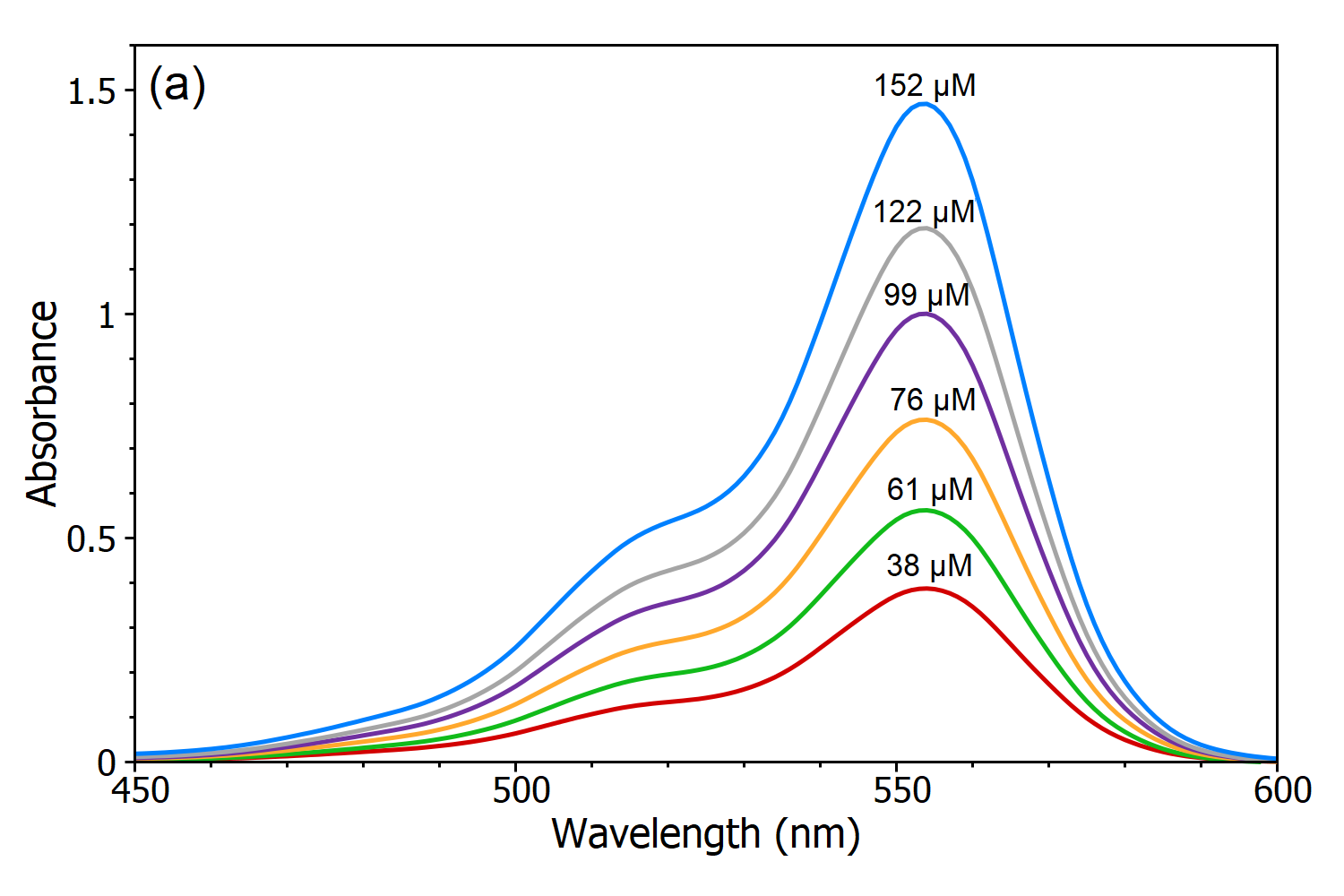
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments

Introduction To Spectrophotometry Ppt Video Online Download
B 7 The Beer Lambert Law Hl Youtube
Absorbance Vs Wavelength According To Beer Lambert Law A Linear Download Scientific Diagram
Beer Lambert Law History Definition Example Calculation
Beer Lambert Law Transmittance Absorbance Edinburgh Instruments
Solved The Beer Lambert Law Is A Linear Relationship For Chegg Com

Beer Lambert Law Transmittance Absorbance Edinburgh Instruments

Beer Lambert Law An Overview Sciencedirect Topics

1 A Schematic Representation For Beer Lambert Law For The Measurement Download Scientific Diagram

Calculating Concentration Using The Beer Lambert Law Worked Example Video Khan Academy

Beer Lambert Spectroscopic Absorbance Principle
Lab 2 Beer S Law And Molar Extinction Coefficients Colorimeter User Manual
Beer Lambert S Law Absorbance Transmittance Spectrophotometry Basic Introduction Chemistry Youtube
Comments
Post a Comment